libre de droit/iStock via Getty Images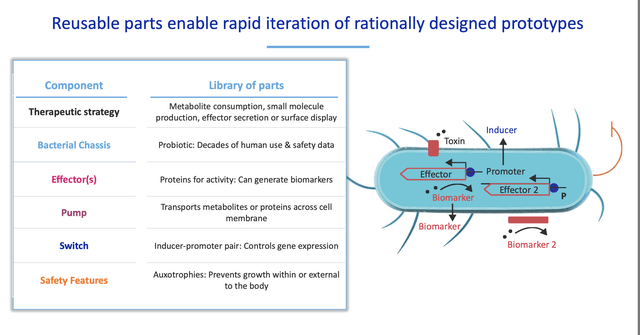
Summary
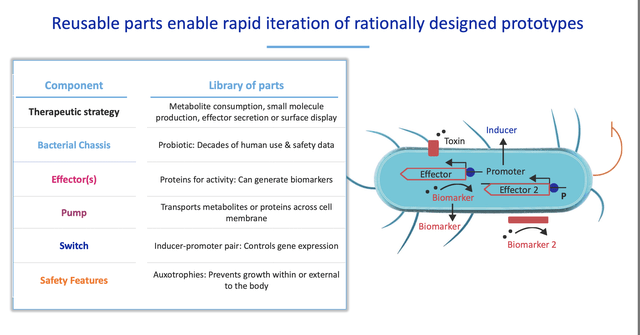
Synlogic Inc.’s (NASDAQ:SYBX) intellectual property is a genetically altered live bacteria which is composed of modular parts that can be engineered to deliver a specific therapeutic effect. The company today produced proof of concept by applying this technology to a rare metabolic disease, PKU, which is a lucrative niche indication with a high level of unmet medical need and a precedent for high pricing. Despite considerable setbacks in the clinic, recent data suggests the company has a valuable asset which will advance to phase 3, SYNB1934. Two data readouts later in 2022 will provide investors with better visibility into the company’s longer term prospects.
Company History
Synlogic was spun out of MIT and uses technology developed by Drs. Tim Lu and Jim Collins. Synlogic was founded in 2014 and became a public company via a reverse merger in 2017. The first candidates utilizes engineered versions of the commensal bacteria Escherichia coli Nissle to consume toxic metabolites in the gut.
There were early setbacks for the company including SYNB1020, a candidate for hyperammonemia, a serious metabolic disease. The program was discontinued after phase 1 data showed a lack of efficacy. The company later focused on diseases with better understood biology and validated biomarkers where reducing a toxic substance is known to positively impact clinical outcomes. SYNB1891 for the treatment of solid tumors was also discontinued.
In addition, the company has sought to develop a candidate for the treatment of inflammatory bowel disease. They first collaborated with AbbVie (ABBV) and now Roche (OTCQX:RHHBY) has partnered and paid milestones to cover development costs. Despite this program first being announced in 2016, this program is still in a pre-clinical stage. Some value may lie in the platform, which may have the capacity to engineer bacteria to perform other functions such as producing small molecules. However, to date, the company has only been able to demonstrate their ability to apply their engineering expertise to one application- consuming toxic metabolites in the gut.
Phenylketonuria
Patients suffering from the rare genetic disease, phenylketonuria (PKU) have an absence or a deficiency in an enzyme called phenylalanine hydroxylase (PAH) which the body utilizes to break down the amino acid phenylalanine (Phe). This amino acid is found in the proteins humans consume as part of a normal diet and the inability to break down Phe causes a buildup in the blood.
Patients have varying degrees of residual PAH enzyme activity but the most severe form of PKU is Classic PKU where patients have little to no PAH enzyme activity. When PAH is deficient or absent, phenylalanine accumulates and is toxic to the brain. Patients can develop severe intellectual disabilities and untreated patients have an average IQ under 50. Other symptoms in PKU patients who are not well treated include seizures, abnormal muscle movements, tremor and eczema. Anxiety, difficulty focusing and headaches may also be caused by high or unstable Phe levels. More subtle intellectual and neuropsychiatric issues may manifest even when patients are treated.
The diagnosis is usually made after routine newborn screening and lifelong treatment is required. PKU biology is well understood- patients who lower dietary Phe intake in turn lower plasma Phe levels and have better cognitive outcomes. Unfortunately, this is a life time endeavor and patients can develop irreversible intellectual deficits if compliance with the diet is poor. It is estimated that there are 34,000 patients in the US and EU who have been diagnosed with PKU. BioMarin (BMRN) estimates that 7,000 patients are currently treated for PKU with medication and BioMarin has the only FDA approved treatments.
A phenylalanine-restricted diet is a mainstay of treatment in both children and adults but controlling plasma phenylalanine levels is extremely challenging. Phenylalanine occurs in most natural proteins and patients generally cannot eat any high protein foods, including meat, seafood, eggs or dairy. Flour based foods, corn and even potatoes contain protein and thus are restricted and must be carefully measured and portions limited. Given this, it is particularly difficult to nourish patients and patients often feel hungry. Children and some adults use specifically designed and expensive formulas (PHE-free amino acid mixtures) in order to nourish themselves. Although the amount of protein allowed varies depending on the severity of the patient’s PKU, most patients cannot eat more than 10 grams of protein a day. Studies suggest compliance with diet is poor given how restrictive the diet is.
Blood phenylalanine levels are monitored and the goal is to maintain levels between 120 and 360 µmol/L. It is estimated that 25% of pediatric patients and 65% of adult PKU patients are not optimally controlled. There is an unmet need for treatments that improve control and also allow patients to eat a more normalized diet.
Approved treatments for PKU
Treatments include Kuvan (sapropterin dihydrochloride), an oral treatment that acts by enhancing the activity of phenylalanine hydroxylase, (PAH) an enzyme involved in breaking down phenylalanine. Patients taking Kuvan must still adhere to dietary restrictions which is extremely challenging. A large portion of PKU patients (~70%) fail to respond but it is recommended that all patients with PKU be given a trial of Kuvan to assess response. 75 % of the 17,000 US patients remain medically untreated and use dietary modifications.
The Pharma Letter described the limitations of Kuvan therapy, stating, “Kuvan is generally more effective for patients presenting with milder forms of PKU as this subset of patients still produce PAH, albeit in lesser amounts and with a decreased efficiency. Kuvan treatment can help to enhance the activity of the residual enzyme in these patients. Even when effective, Kuvan must be combined with a phenylalanine-restricted diet to maintain optimal plasma levels.”
The treatment effect of Kuvan (mean percent changes of -29% in one study) allows patients to eat only a few additional grams of protein per day and thus patients are still burdened by maintaining a Phe restricted diet. Kuvan (before becoming generically available) cost between $57,000 and 200,000 per patient per year. Kuvan sales were $500 m before it became generically available.
Palynziq, (pegvaliase-pqpz) which is also made by BioMarin, is a daily injectable that contains a modified form of the PAH enzyme and allows some patients to eat a less restrictive diet and discontinue medical formula. It is only for adults and not recommended for patients over 65. Most patients in the pivotal trials were not on a low Phe diet but 60% of patients achieved Phe levels ≤360 μmol/L. Annual revenues are estimated at $300 million.
However, the product insert for Palyniq contains a warning because clinical trials showed that 9 % of treated patients experienced anaphylaxis. Patients are prescribed epinephrine and prescribing is restricted to specialists who have been trained to manage this risk. Treatment related adverse events led to a discontinuation rate of 15% with 70% of patients reporting arthralgia (back pain, neck pain, and musculoskeletal pain), 47% reporting skin reactions and 47% reporting headaches. A post approval study reported that anaphylactic episodes were reported by 21.6% of patients in a PKU clinic with only approximately 35% of patients maintaining Phe levels in the target range. This suggests real life efficacy (35% of patients maintaining target Phe levels vs 60%) and safety (21% anaphylaxis rate vs 10%) are much worse than observed during the clinical trials.
Many PKU patients are not well served by Kuvan and Palyniq and there is a real need for safer and more effective treatments, including ones which allow patients to liberalize their diet. In a 625 patient survey conducted by the National PKU Alliance, 91% of surveyed PKU patients think developing new treatments is important. Only half of the patients surveyed were able to maintain their Phe levels in the range between 120-360 µmol/L. Patients cited that key attributes of an ideal therapeutic include allowing patients to increase their intake of natural protein and allowing them to discontinue or reduce the use of medical foods such as formulas. Patient preferences were for oral treatments that could be self administered and 88% of surveyed patients would be interested in a daily “probiotic” to treat their PKU.
SYNB1618 and SYNB1934 for PKU
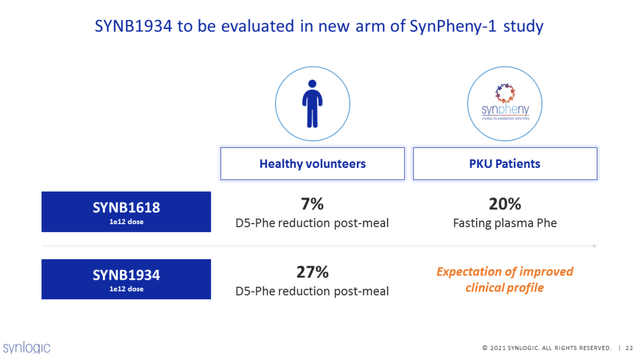
Synlogic has developed SYNB1618 and SYNB1934 for the treatment of PKU. Synlogic scientists genetically altered a common gut bacteria with a Phe transporter to bring Phe into the bacterial cell where it has been engineered to break it down. This is a non-replicating strain of a commensal bacteria that is cleared from the body and can be orally administered with meals. In testing in human volunteers, SYNB1934 appeared to be significantly more effective at Phe reduction.
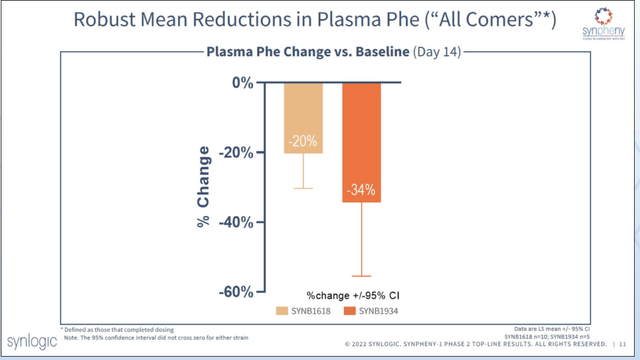
Results from the Phase 2 Synpheny-1 study of SYNB1618 and SYNB1934 in patients with PKU were announced today. All patients enrolled had Phe >600 despite diet and/or Kuvan treatment. The primary endpoint is the change in area under the curve (AUC) of plasma levels of labeled D5-phenylalanine (D5-Phe) after a meal challenge before and after the treatment period, which is a measure of the ability to consume Phe. The study included a dose-ramp over 15 days of treatment, with days 7 through 14 at the constant dose of 1×1012. When “all comers” were included, the day 14 mean change from baseline in fasting plasma Phe was -20% for SYNB1618 and -34% for SYNB1934. Among responders to treatment, there was a 42% reduction in Phe levels. Data in healthy volunteers was highly correlated with results in PKU patients. Moreover, these results included data from patients who were already taking Kuvan at baseline. This suggests 1934 can be used in combination treatment. Adverse events were all mild to moderate and predominantly GI in nature with no SAEs. Better titration regimens will be attempted in the phase 3 trial to improve tolerability.
Based on this data, SYNB1934 will advance to phase 3. Synlogic’s candidates can potentially be used as mono therapy and in combination with other therapies (adjunctive) to help patients maintain Phe levels in range while allowing them to liberalize diet. Given the mechanism of action (consuming Phe), efficacy should not be dependent on the presence of PAH and a wide range of PKU patients could potentially respond to treatment. In addition, a commensal bacteria that clears the system after dosing may have safety advantages over other potential therapies.
Investigational Stage Treatments for PKU
There are investigational stage gene therapies designed to provide patients with functional copies of the PAH gene. BMN307, (BioMarin Pharmaceuticals), is an AAV5 gene therapy for PKU. The FDA placed a clinical hold on BioMarin’s program in September of 2021 because 6/7 mice given the highest dose of BMN-307 developing liver cancer. The FDA is requiring additional animal and/or pre-clinical studies to better assess the risk to human subjects. In the past, the FDA has sought to ascertain whether AAV vectors integrate into the genome which is viewed as potentially harmful. As extensively discussed in an article in the journal Nature, AAV and lentivirus gene therapy have been associated with safety concerns from cancer to immune reactions. Durability is also a concern as many patients are diagnosed as infants.
Homology Medicines (FIXX) has a PKU gene therapy and an in vivo gene editing therapy (HMI102 and 103 respectively), under development. HMI102’s phase 1/2 data release contained insufficient detail to evaluate the treatment effect. The company appears to be moving forward with HM103 as well. Pfizer has made an investment in Homology and has the rights of first refusal to their PKU program suggesting they see promise in these potential one dose treatments. Homology used a higher dose than BioMarin and liver enzyme elevations were observed. The FDA put a clinical hold on the program in February of 2022. On a broader note, the FDA has been signaling a higher bar for safety and durability in gene therapy when there are available treatments.
PTC Therapeutics (PTCT) acquired a PKU program from Censa Pharmaceuticals which has showed promising efficacy and safety. PTC923 is designed to increase the activity of the PAH enzyme. A phase 2 study enrolled 24 PKU patients regardless of severity of disease. 11 of the enrolled subjects had classical PKU (considered the most severe form of PKU) and 13 non-classical PKU . Each subject tested 2 doses (20 and 60 mg/kg/day) of PTC-923 and compared it to their response to Kuvan (20 mg/kg/day) with washout periods in between each treatment. All participants continued on a low protein, Phe restricted diet.
Both doses of PTC923 provided reductions in blood Phe. The overall blood Phe reduction for Kuvan in this study was −91.5 μmol/L vs 206 μmol/L for high dose PTC923. The subgroup of patients with classic PKU showed a reduction of plasma Phe levels of − 150.8 μmol/L. 50% of the subjects at the higher dose achieved blood Phe <360 μmol/L versus 42% of subjects receiving sapropterin dihydrochloride. Nervous system disorders and headaches were the most common side effects impacting 16 % of participants. Treatment was generally well tolerated with no serious adverse events. The APHENITY Phase 3 trial of PTC923 for PKU is underway with results anticipated by the end of 2022.
Nestle licensed CDX-6114 an acid stable phenylalanine ammonia lyase (PAL) enzyme that is oral and can consume Phe in the small intestine. Phase 1a has been completed but no further studies have been initiated.
Risks
Data for SYNB1934 from the phase 2 Synpheny-1 study included 15 patients. Given PKU is a rare disease, the FDA requires smaller studies for regulatory purposes and only one phase 3 trial is the likely requirement based on precedent. However, for investors, assessing the profile when only 15 PKU patients were tested in phase 2 poses risk especially as there is a great deal of variability between how patients respond to treatments in PKU. Phase 3 results could vary considerably from phase 2 results and investors face the risk of phase 3 data which is not clinically meaningful and/or does not meet the threshold for regulatory approval. In addition, while Kuvan and Palyniq sales have helped to establish the market ($800 m), without knowing the clinical profile and how SYNB1934 will fit it into the treatment paradigm, a peak sales estimate is unlikely to be accurate. SYNB1353 for homocystinuria and SYNB8802 for enteric hyperoxaluria are very high risk programs for investors given their early stage.
Financials
At the end of last quarter, the company had $106 million of cash on hand which they project is sufficient to sustain operations into 2024. Cash burn last quarter was approximately $16 m suggesting the company may need a capital raise in late 2023. The company is funding potentially 3 clinical stage assets but they do receive milestone payments from Roche for the IBD program. The current market cap is $74m but the $106 m in cash on hand is likely to be entirely used and additional capital is likely to be required as there is long path ahead (2-3 years) before commercialization of SYNB1934 could occur.
Conclusions
The data released today was sufficiently positive to move to phase 3 which the company expects to initiate in Q123. Results were largely what investors may have anticipated and consistent with the treatment effect in healthy human volunteers. Data suggests SYNB1934 may be a safe, effective treatment for PKU and that it may be useful in combination with other treatments. After the data release today, Oppenheimer raised the price target to $8 and maintained an Outperform rating. The most important competitor to watch is PTC Therapeutics which appears to have a safe, effective PKU treatment. PTC923 may become commercially available in late 2023 or early 2024 if data is positive.
Additional early stage data for SYNB1353 for homocystinuria and SYNB8802 for enteric hyperoxaluria is due by year end. If these experimental medicines show safety and efficacy, Synlogic will be well positioned with multiple assets in the clinic in indications where premium pricing is likely given the serious nature of the diseases. In this difficult market for biotech funding, Synlogic has Orbimed and RA Capital each owning approximately 9 % of the company suggesting they may have funding options to bring these assets forward. Investors may wish to wait and see how data for SYNB1353 for homocystinuria and SYNB8802 for enteric hyperoxaluria reads out. If positive, the full pipeline may make the company a more attractive investment in this difficult market for small cap unprofitable biotech companies.


Be the first to comment