Afrezza Was Given FDA Approval on June 27th, 2014:
We are now into the seventh (7) calendar year that MannKind (MNKD) has had FDA approval for Afrezza being sold in the United States. Much has happened with the company during this interim period. However, the one critical event that is needed to keep MannKind funded as a manufacturing and marketing entity is their ability to obtain initial users and then maintain patients as users by refilling Afrezza in a meaningful way. Since Afrezza has been on the market, the ensuing years have produced results that imply MannKind’s annual expenses being in the $100,000,000.00 range. The latest year, 2019, MannKind reported the highest annual net revenue for Afrezza sales being a mere $25.3 million. Based on just these two metrics we can see net sales revenue for Afrezza is only creating one-fourth of the net profits needed to cover the annual expenses incurred for operating MannKind. The major obstacle creating this shortfall is the inability for achieving the needed sustainable revenue flow and this has been well documented in the clinical data generated for the three NDAs filed with the FDA seeking their approval. The same issue from this pre-launch of the product continue to manifest and amply the issue—those who were initial users in the clinical trials and now those initially adopting Afrezza as their insulin prescription—these individuals are refusing to continue using Afrezza after trying the product. This shortfall reality can be seen in three visible and identifiable examples of this issue:
|
Initial Key Dates for Critical Data Points: |
|||||
|
Date: |
Data Point |
||||
|
421 |
10/12/2015 |
Hitting the 400 Level for Weekly NRxs |
|||
|
404 |
12/27/2018 |
Hitting the 400 Level for Weekly Refills |
|||
|
$1.117M |
12/21/2018 |
Hitting the $1.0 Million in Revenue |
|||
|
Latest Weekly Data for Each of the Above: |
|||||
|
353 |
2/28/2020 |
Week’s NRxs |
|||
|
396 |
2/28/2020 |
Week’s Refills |
|||
|
$1.20 M |
2/28/2020 |
Week’s Revenue |
|||
These three data point categories clearly reflect that now, well into the sixth calendar year that Afrezza has been on the market, weekly new prescriptions are 68 less than the 400-level milestone achieved in 2015—more than 5 years ago. But more revealing is the fact that with the latest weekly refill number, the refills are less than what was being achieved in 2018. Based on the fact refills should be refilled within a maximum time of 90 days (3 months) we know that in 2015, in 48 weeks there were approximately 13,000 new prescriptions filled for Afrezza. Therefore, in 2016, spreading these 13,000 new prescriptions that normally needs refilling, this translates into 250 weekly refills just for the 2015 number, 250 for the 52 weeks of 2017, 250 for the 2018 weeks and 250 for 2019. Now consider for example purposes, in the year of 2016, MannKind secured another 13,000 new users and this would mean the refill for 2017 should be 500 each week (250 from 2015 + 250 from 2016.) Now extend this example through 2017, 2018, 2019 and now into 2020. MannKind takes the time to point out that they are growing the prescriptions on a year-over-year comparison, but in my example assume the new prescriptions have remained flat with 2015 results, the subsequent refills now should be running 1,250 each week. However, the fact is, we are seeing weekly refills averaging in the 300s. Just for refills getting into alignment with a miniscule 13,000 (2015) accomplishment, refills will need to grow 4-times the current rate of refills just to gain parity with what should be normal refilling patterns.
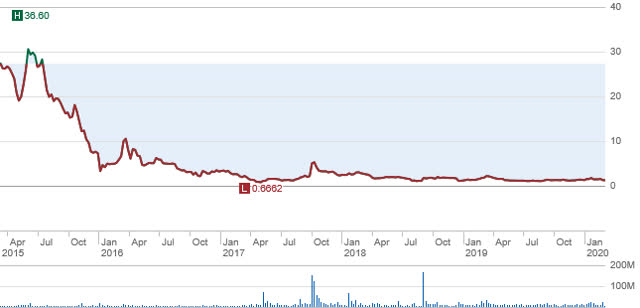
The following is a chart reflecting the trading pattern for MannKind’s stock. This chart reproduces the trading from 2015 through the current time frame. The failure to secure needed refills helps explain why the stock is now trading for around $1.25. However, when one adjusts this price for the previous 1:5 reverse split, the holders of this stock since 2015 own a stock valued at around $0.25 per share. The refills issue is the major obstacle needing a resolution found, but I will give further examples in this article where I will share the causation for this shortfall being experienced. But first MannKind must secure a growing list of initial users and they must refill on a timely manner their Afrezza prescriptions.
(Sourced from Schwab)
The Latest Amazing Admission from MannKind in Their 2019 Annual Report Filings -A Training Wheel Device – #1:
The global inhalation and nasal spray drugs are projected to generate $44.3 billion in revenue by 2025. The latest numbers indicate there are nearly 100 such drugs on the market with the potential for the actual numbers soon expanding dramatically as drugs lose their patent exclusivity. With such a massive market already in place I find it surprising MannKind appears to be the only drug company having an inhaled product needing a “training wheels” device in order to teach patients how to properly use their inhaler. The dropout rate for Afrezza users has been an issue since potential users were enrolled in FDA sanctioned clinical trial—nearly two decades ago. Now well into six calendar years being on the market and MannKind suddenly thinks a training aid will solve the problem that appears not to impact what is already a massive market where an inhalation device is necessary to deliver a drug to a human patient. This new training device appears to be unique and only needed by MannKind’s product!
The following is quoted directly from the recent 4th-Q and Full-Year (2019) Annual Report on February 26, 2020:
“We think about how we’re going to get there. Number one is our safety in PK in pediatric patients. This allows us to explore how to get to Phase III as the pediatric market is about 10% of the rapid-acting market and represents over $1 billion opportunity for Afrezza. As we think about getting standards of care change and adoption, that’s around building prescriber confidence and educator confidence. And I’m proud to say these next 2 bullets around Afrezza BluHale were data sets that demonstrated last year that using a training device like BluHale to show proper inhalation will build someone’s confidence and reduce teaching time, which ultimately reduces the administrative burden in these offices who are overwhelmed with paperwork these days. BluHale PRO will be the name of our health care professional addition that we will launch here in Q2.”
Very clearly, the CEO is thinking about how they are going to get there with their Number One priority wrapped around the safety in PK, using adult data to support safety issues in pediatric patients. If MannKind had merely stopped with that singular point, it would have been wise on their part. But they didn’t stop because the presenter then went to the next bullet point and stated their BluHale product as being merely a training device to show users how to use their inhaler. It is clearly implied the BluHale product is being developed to ameliorate the doctor’s office staff from being overwhelmed with teaching a patient how to use the inhaler, and thus reduce the administrative burden for their staff. Now five years plus in trying to market Afrezza to medical prescribers and their patients, the 75% drop out rate is being addressed with a product that even MannKInd admits they have been working on for at least a decade and it still isn’t ready for launch. Already having an issue getting insurance companies to cover Afrezza without applying restrictive limitations for the product and now apparently MannKind thinks adding another component that no other drug manufacturer finds necessary to market their inhaled drug, my guess this isn’t going to enhance the potential for insurance companies and distributors being enthused with such a teaching aid.
2019 Annual Report Amazing Admission – Receptor Life Sciences – #2:
On January 21 st, 2016, MannKind announced their partnership deal with Receptor Life Sciences— “We are pleased that Receptor Life Sciences has selected our formulation and delivery technology to advance its portfolio of innovative products –Matthew Pfeffer.”
We know from checking the FDA’s data that Receptor hasn’t filed a request for any human clinical trials utilizing their innovative products. And now we know the status of this partnership by checking what MannKind states about this four-year-old partnership in their 2019 annual report.
“In January 2016, we entered into a collaboration and license agreement with Receptor Life Sciences (“Receptor”), pursuant to which Receptor is responsible for the development, manufacture and commercialization of inhaled formulations of certain cannabinoid compounds utilizing our technology. We have been informed by Receptor that it has evaluated safety, tolerability and pharmacokinetics of prototype powders in initial clinical trials; however, data from these clinical trials has not yet been published or made available to us.”
MannKind has a partnership deal with a company where a principal had a long work history and participated in the development of MannKind’s technology. Now this company tells MannKind they have discovered critical data points, but they apparently aren’t willing to share this information with MannKind! I have difficulty understanding that a partnership has been formally agreed up and now critical data that should dictate the future of this partnership and they opt not to tell MannKind if there is any merit to five years of work and time. MannKind deserves having such critical information so they can prepare to deliver their part of the drug development being suggested for going on five years? This stalemate in what has been previously promoted as a revenue stream for MannKind, it appears we might be facing the realization that just another previous promise is not coming to fruition!
The Most Amazing Admission in the 2019 Annual Report-Ignore Between 30-40% of the Potential Market -#3:
But of all the admissions in the recent annual report, I find this one the most amazing. Based on the historical record of this issue, the fact that other diabetic drug developers know the critical needs for this market segment, and they have product that include this market segment that MannKind feels they must avoid. Looking at this issue as a whole, Mannkind fully realizes they have a dosing issue they haven’t been able to resolve with Afrezza. Basically, they are waving red flags with what they are proposing to the FDA. But before going into the latest details allow me the opportunity to give the historical unfolding of this issue.
When MannKind signed their partnership deal with Sanofi (SNY), June 26, 2015, I went on record with my first SA article where I outlined my reservations about what I considered a one-sided deal where Sanofi had dealt themselves a royal flush hand. My naysayers attacked my position with the claim that Sanofi was the perfect and most ideal partnership that MannKind could have. Then when Sanofi walked away from the deal, the same naysayers assailed Sanofi with the claim they had no intention for promoting the merits and benefits of Afrezza. And now in the latest annual report we are finally seeing MannKind address the very issue that Sanofi deemed necessary for success on August 15th, 2015. It was on this date in 2015, Sanofi had filed with the FDA their agreed upon protocol for a pediatric study using Afrezza. So right up front in the new partnership Sanofi had committed to a pediatric clinical trial, atrial they knew they must conduct if the wanted success with the product. In my opinion Sanofi realized this market would be key for growing the revenues for Afrezza.
The first thing to note about this critical and needed trial can be seen in the official title given for this Sanofi led effort –“ Open-Label, Single-arm, Multiple-dose safety, titration, and Pharmacokinetic trial of Afrezza in pediatric patients age 4 to 17 years with Type 1 Diabetes.” But now we know that only a few months later, December 2015, Sanofi had seen the results of the adults using Afrezza and they opted to walk away and take a massive loss on the money they had invested in the partnership. But in short order, Sanofi made a full return to MannKind, the control of all aspects of the product. This included the rights for the already approved by the FDA clinical trial for a pediatric study for patients age 4 to 17 years with Type 1 Diabetes.
At the beginning of 2016, MannKind had their hand’s full, trying to conclude the Afrezza asset’s transfer back from Sanofi and developing a marketing plan to turn around the dire situation they faced. And where credit was due and for generating a lot of excitement for shareholders was the mid-2016, announcement MannKind was in a collaboration with the Juvenile Diabetes Research Foundation, the JDRF.
“The next big thing that I think is critically important for those of you who know about diabetes is that a lot of Type 1 patients are diagnosed as children, and they continue to go on through their lives needing insulin. So getting our pediatric protocol off the ground and running and getting patients enrolled is one of my top priorities,” Castagna said at a July 12th investor forum.”
But back in September 2016, after MannKInd had announced their collaboration with the Juvenile Diabetes Research Foundation – JDRF –the senior researcher for the JDFA made the following statement –
“ JDRF’s senior research vice president Dr. Steven Griffen said in a telephone interview that it makes sense that Mannkind would want to do pediatric clinical trials. It was unusual for the FDA to clear a new drug without evidence to establish its effectiveness for pediatric use. “New therapies typically require a pediatric program,” he said. The challenge in these studies will come with translating doses from injectable insulin to appropriate inhaled doses, according to Dr. Griffen. With pediatric patients, it will involve strict attention to variables such as mealtime carbohydrate load, timing of meals and snacks, and activity level. Clinical studies on pediatric use should be underway in the fall of 2016. When Mannkind plans to submit Afrezza for expanded use is not clear now, said Dr. Griffen. “MannKind owns the timeline, and I’m sure that MannKind intends to take the time necessary to do it right,” Dr. Griffen said.”
So in 2016, after taking time to form an alliance with the world’s leading juvenile diabetes research organization, where it appears that both partners were in perfect agreement that the needed pediatric clinical trials would be the next priority for enhancing the success of Afrezza in the all Type 1 diabetes age 4 and upwards. The CEO was very clear in 2016 when he stated—“The next big thing that I think is critically important for those of you who know about diabetes is that a lot of Type 1 patients are diagnosed as children —-“
But in a total surprise—we suddenly got nothing but silence and only money being spent funding promotional videos by individuals that weren’t exactly household names and decals on race cars going nearly 200 miles an hour around race track. But no such funding for the very item that had been identified as being critically important!
For the rest of 2016, all of 2017, all of 2018, all of 2019, we heard nothing from this issue. Only now, during the recent 2019 annual report in February 2020, we get a new commitment for the promised 2016 clinical trial with pediatric patients. But we didn’t get what had been cleared with the FDA way back in 2015.
The following is quoted directly from the recent 4 th-Q and Full-Year (2019) Annual Report on February 26, 2020:
“We think about how we’re going to get there. Number one is our safety in PK in pediatric patients. This allows us to explore how to get to Phase III as the pediatric market is about 10% of the rapid-acting market and represents over $1 billion opportunity for Afrezza. As we think about getting standards of care change and adoption, that’s around building prescriber confidence and educator confidence. And I’m proud to say these next 2 bullets around Afrezza BluHale were data sets that demonstrated last year that using a training device like BluHale to show proper inhalation will build someone’s confidence and reduce teaching time, which ultimately reduces the administrative burden in these offices who are overwhelmed with paperwork these days. BluHale PRO will be the name of our health care professional addition that we will launch here in Q2.”
Promoting the plans to enter a new $1 billion opportunity, even after waiting for five (5) years, is something that should perk up investor’s ears and eyes. That is if you ignore what the fine details of their new effort with the FDA is based on.
“ So on the pediatric, I mean we were very excited to get alignment with FDA directionally on finalization of the part 1 of the 2 parts for approval. Part 1 was just confirming that our PK results meet the criteria for the FDA for the 8- to 17-year old. And then the second part of that alignment with FDA was that we’re going to forgo a 4- to 7-year-old label indication, and they’ve aligned directionally to those 2 requests.”
Basically, MannKind has approached the FDA and is seeking approval from them is permission to eliminate nearly 35-40% (age 2 to 8) of the potential pediatric patients that are Type 1. I haven’t taken the time to review all insulin products that are FDA approved, but I did look at FIASP, the latest FDA approved fast acting insulin product. I also looked at Novolog and found Novo Nordisk (NVO) has pediatric approval for patients from age 2 and upwards for all ages. Novo Nordisk further states they follow the ADA’s guidelines for pediatric patients. This has left me to ponder—why has MannKind opted to eliminate all Type 1 patients younger than age 8, when major competitors had the foresight to seek approval for pediatric patients during their initial clinical trials. The only conclusion that I have been able to arrive at, and based on the fact MannKind has discovered adults aren’t having success with their dosing regimen, expecting a two-year old to handle this task would be out of the realm of possibility. It is Plain and Simple! Dosing Afrezza has been a historical noted issue that now MannKind is admitting and seeking a solution with a secondary product that has been in development for years—but by doing such, they are merely amplify the fact that competing products aren’t faced with this critical issue.
Final Comments:
Five years into marketing Afrezza the weekly new prescriptions since January 2019 through to the most recent week has average 310 prescriptions. The 300 weekly level was achieved in 2015, and now five years later it has flat lined for the last 50+ weeks. But more telling is the overall growth in new prescriptions and refills combined:
- (2015—19,604 TRxs)
- (2016 –35,371 TRxs cumulative—increase of 80%)
- (2017 –52,988 TRxs cumulative –increase of 50%)
- (2018—80,622 TRxs cumulative – increase of 52%)
- (2019 – 116,023 TRxs cumulative –increase of 44%.)
Seeing that the last full year (2019) showed that total refills had declined to a rate of sequential growth of only 44%, and with the shortfall already failing to generate more than a net annual revenue of $25 million, it brings up the question of how many more years can MannKind secure funding for operational basic needs.
MannKind continues to promote they are seeking Standard of Care – SOC – recognition for Afrezza. However, in admitting that potential users will need a training device for learning how to dose Afrezza, and now admitting Afrezza apparently isn’t suited for all of the pediatric patients that competitors serve, in my opinion this isn’t the course of action to earn Standard of Care recognition for Afrezza.
I have always stated it is my wish for Afrezza remaining available for those patients who need options in meeting their healthcare needs. Maybe a viable option is finding a successful and major drug company willing to take over the management of Afrezza and pay MannKind a royalty income for any revenue generated. Think of the adage—”A bird in the hand is worth two in the bush.” Any revenue stream that has no expense in creating the product is better than money flowing only one way—and that way is currently out of MannKind’s bank account. This will free MannKind to continue their efforts in developing and using their technology. Based on the history for the lack of success in creating a product line, this will not be an easy task. But all should agree—the current path hasn’t been successful. Giving the current management credit for one thing—an amazing ability to secure funding to keep the doors open and the lights on. This doesn’t include taking Videx up on their recent efforts to form a partnership with MannKind. People need to re-read the White Paper documents and note the key details and basis for what they contend is the road map to success—especially considering the issues raised and highlighted in this article. The key is what I stated—a successful and major drug company. And finally, they don’t need deals like the one with United Therapeutics simply because I’m still puzzled why United has stated they don’t necessarily intend to use MannKind as the manufacturer of their joint product. As it stated now–MannKind’s role is still creating the product for use in the current clinical trials and then United has the option for taking over the manufacturing. MannKind needs for the Danville plant operating and put out a product generating revenue based on manufacturing the product.
Our nation is on the cusp of a catastrophic pandemic that has world-wide applications to our economic viability—find funding for unprofitable corporations will not be an easy task for any executive team—my sincere hope is that MannKind’s haven’t lost their ability in this area. Being successful one more time and they will have earned their pay package!
Good luck with your future investing decisions!
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.


Be the first to comment