alvarez/E+ via Getty Images
Lineage Cell Therapeutics (NYSE:LCTX) is a pre-clinical and early clinical stage drug development company that specializes in cellular based therapies. Lineage develops specialized cell types that support and augment poorly functioning native cells. These cells are mass produced from embryonic stem cells and stored frozen in liquid nitrogen. The cells can then be thawed and implanted into patients on an as needed basis. They currently have in their pipeline cells that activate the immune system, treat spinal cord injuries, and repair/replace cells contained in the eye. In this article I want to discuss their cellular treatment for Dry Age-related Macular Degeneration [AMD] with Geographic Atrophy [GA], and speculate on the future of their newly developed line of photoreceptor cells.
Dry AMD with GA is an irreversible disease with no currently available treatment. Typically, AMD with GA starts with a degradation of the retinal pigment epithelium (RPE) cells. RPE cells are responsible for creating a microenvironment in which the photoreceptors can thrive; because of this, once the RPE cells start to degrade, the photoreceptors will soon follow, which will ultimately lead to blindness.
Cross section of the Retina (UCL INSTITUTE OF OPHTHALMOLOGY)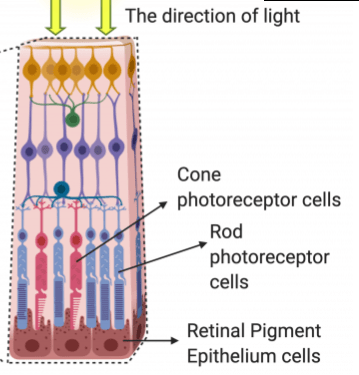
In Lineage’s clinical trial for dry AMD, Lineage injected their proprietary formulation of RPE progenitor cells (OpRegen) directly into the eye. These progenitor cells ultimately differentiated into fully functioning RPE cells that augmented the function of the patient’s naturally occurring RPE cells.
An initial trial of lineage’s RPE progenitors consisting of twenty four patients has recently been completed. Twelve of the twenty four patients were legally blind. In the legally blind group OpRegen did not show any efficacy. However, in the group that was not legally blind, 75% of the patients had an improvement in their vision. Of the clinical trials that I’ve been able to look at, only one other drug has been able to achieve this level of success (discussed below).
“OpRegen has been well tolerated to date, with no unexpected adverse events“.
Summary of Safety Results:
- All 24 treated patients reported at least one adverse event [AE] and at least one ocular AE
- The majority of AEs reported with OpRegen were mild (Cohort 1-3, 87%; Cohort 4, 93%), and the immunosuppressive regimen was well tolerated
- Ocular AEs observed with OpRegen were mainly related to the surgical procedures used for subretinal delivery, with the most common being conjunctival hemorrhage/hyperemia (n=17) and epiretinal membrane (n=16)
- One patient discontinued the study due to an AE that was unrelated to treatment
- No cases of rejection, acute or delayed intraocular inflammation, or sustained increases in intraocular pressure following OpRegen subretinal delivery have been reported
Hoffmann-La Roche
At the end of 2021 Lineage sold the rights to OpRegen (renamed RG6501) to Genentech, a subsidiary of Hoffmann-La Roche (OTCQX:RHHBY). In the agreement, Lineage will remain responsible for RG6501 production, and Genentech will take control of the clinical trials. In return, Lineage received a $50 million upfront payment. In addition:
Lineage is eligible to receive up to an additional $620 million in certain developmental, regulatory and commercialization milestone payments. Lineage is also eligible for tiered double-digit percentage royalties on net sales of RG6501.
Since Lineage does not own all of the IP that underpins RG6501, $21 million of the $50 million had to be turned over to various IP holders. Leaving $29 million for lineage. The money was paid in one lump sum on 12/21. However, Lineage is accruing the income on a milestone completed basis. This is perfectly acceptable on both a GAAP and tax basis but creates a distortion when earnings are used to make investment decisions.
A redacted version of the Roche agreement can be found here. In the agreement, Roche has committed itself to conduct large scale clinical trials of RG6501. The first of which started in mid December of 2022. Sixty patients will be treated in this trial. Roche will follow their progress for seven years. Missing from the redacted document is specific information on the financial terms and on the IP that is being transferred to Roche. Since this agreement with Roche is by far their most important asset, the lack of transparency is somewhat disappointing.
PNC1
About a year ago, Lineage announced that they had successfully turned embryonic stem cells into photoreceptor cells (PNC1). However, beyond their existence, no information on the cell line was provided. They have not even identified the specific type of photoreceptor (there are three different types). Nevertheless, despite the lack of information, I suspect that there is a strong potential for synergy between these cells and Lineage’s RPE progenitor cells.
For example, in the recent OpRegen trial that Lineage completed, legally blind patients did not see any improvement in their eyesight. My guess (and keep in mind that my background is not in biology) is that this is because the photoreceptors in the blind patients had died and that restoring the microenvironment with RPE cells was pointless. However, if you inject RPE cells to restore the microenvironment and healthy photoreceptors to replace the ones that had died, you may be able to restore the vision of previously blind patients.
Competition
The table below is a list of clinical trials for Dry AMD with GA that I have found. There are very likely many more that I have not been able to find (see note 1). There are too many of these trials to discuss in this article. Five of them, however, are noteworthy. The first drugs to reach the market will be ALK-001, APL-2/Pegcetacoplan, and Zimura/Avacincaptad pegol. These drugs slow the progression rate of the disease but do not reverse it. Another drug worth mentioning is FHTR2163. FHTR2163 is being developed by Roche, the same people that purchased the rights to Lineage’s OpRegen. I assume this means that they feel that OpRegen is a superior product.
By far the most noteworthy drug on the list below is CPCB-RPE1 from Regenerative Patch Technologies. Like OpRegen, CPCB-RPE1 uses RPE cells; also, like OpRegen, it appears to be able to reverse the course of GA. The results for CPCB-RPE1 are actually very impressive (12 month results, 34 month results). However, from what I’ve read, my (uneducated) guess is that CPCB-RPE1 is less amenable to mass production than OpRegen and will therefore be a more expensive product.
|
Clinical stage |
Delivery method |
Adverse events |
|
|
ALK-001 (Alkeus Pharmaceuticals) |
Phase 3. Estimated completion date: 9/30/23. |
Daily oral administration for 24 months |
|
|
APL-2/Pegcetacoplan (Appellis) |
Phase 3. Estimated completion date: July 2025 |
Injection into the eyes monthly or every other month for 5 years |
|
|
CPCB-RPE1 Implant (Regenerative Patch Technologies) |
Phase 2 Completion date June 2023 |
Surgical procedure |
|
|
FHTR2163 (Genentech/Roche) |
Completed phase 1. Two Phase 2 trials in progress. Estimated completion dates: 12/15/23 and 12/19/25 |
Multiple injections into the eyes space 4 to 8 weeks apart. |
46% of the patients had an adverse event. |
|
Zimura/Avacincaptad pegol (IVERIC bio) |
Two phase 3 studies. Estimated completion dates: July 2023 and January 2025 |
Injections into the eyes monthly or every other month for two years. |
|
|
AAVCAGsCD59 (HMR59) (Johnson & Johnson) |
Completed phase 1. Phase 2 being planned. |
Injection into the eyes |
Mild inflammation |
|
ANX007(Annexon Biosciences) |
Phase 2. Estimated completion date: 12/31/2023 |
Injections into the eyes every other month |
? |
|
AVD 104 (Aviceda Therapeutics) |
Waiting for FDA approval to start clinical testing. |
? |
TBD |
|
CNTO-2476 Palucorcel (Johnson & Johnson) |
Phase 2 completion August 2022 |
single subretinal injection |
|
|
Elamipretide (Stealth Biotherapeutics) |
Phase 2 Estimated completion date: June 30, 2024 |
Daily injections for 52 weeks |
mild |
|
GT005 (Gyroscope Therapeutics) |
Three phase 2 studies are currently ongoing. Completion dates are 02/28/24, 10/31/25, and 01/19/27There is also an ongoing follow-up study that ends on 09/16/28. |
A single injection into the eyes |
? |
|
NGM621(NGM Biopharmaceuticals) |
Phse 2. Estimated completion date: April 2023 |
Injections into the eyes every 4 or 8 weeks |
? |
Speculations and Conclusions
In OpRegen’s phase 1 clinical trial, 12 non-blind patients were treated. Twelve months later, their average gain in visual acuity (visual acuity refers to the number of letters that the patients are able to read on an eye chart) was 7.6 letters; a quarter of these patients showed a 15 letter or greater gain.
These results sound very impressive. However, generally, drug trials require large sample sizes in order to rule out the possibility that the outcome was a result of uncontrolled random factors. The term for this is statistical significance, and with only twelve patients in the trial, it is very difficult to conclude that the results are statistically significant.
In cases like this, statistical significance can still be determined, but to do so would require some very good statistical data on how dry AMD with GA normally progresses over time in patients that have not received any treatment for their condition.
I’ve attempted to find some statistics on the likelihood of seeing an improvement in untreated patients, but haven’t been able to find any really good data. The best I could find was this study here. In this study 95 patients, which is still too small to be statistically significant, were followed for five years. Despite the small size of the trial, the results are still insightful. In the study, two people actually showed an improvement in their visual acuity. However, neither these patients nor any of the other patients showed any reduction in the size of the geographic atrophy. This study strongly suggests that patients suffering from GA do not recover. And by inference, it can be concluded that the OpRegen results are likely to be statistically significant.
A similar argument can be made with the 15 patient CPCB-RPE1 study. In the CPCB-RPE1 study, one eye was treated with RPE cells, while the other eye was used as a control. This makes the above argument even stronger for the CPCB-RPE1 study.
Combining the two studies provides a very believable likelihood that RPE cells provide an effective means for treating dry AMD with GA.
To be clear, I’m not saying that the results for the OpRegen or CPCB-RPE1 have reached a level of statistical significance. What I am saying is that the results of these trials are very impressive. And that these results are far stronger than what would normally be expected for such small sample sizes.
Final Comment
I really like this company. In addition to their vision related products, they have developed cells for coating nerve fibers with protective “myelin sheaths”, Dendritic cells for modulating the immune system, and natural killer cells that are used by the immune system as a first line of defense (for additional details see my previous article).
Despite all this I cannot rate them as a buy. For one, key elements of the Roche agreement have not been made public. The Roche agreement is one of the main reasons that I’ve been following this company, but there are just too many unknowns in agreement.
Another issue that I have at the moment is that I suspect that the market psychology is changing from an attitude of “buy anything that sounds techie” to one of “show me the money”. This does not bode well for companies like Lineage.
For now, I’m waiting for events like further stock price erosion, milestone payments from Roche, and an update on the RG6501 trial.
Note 1.
I used the clinicaltrial.gov database to search for ongoing clinical trials. Clinicaltrial.gov is the largest of the clinical trial registration databases. There are other smaller ones which I have not searched.


Be the first to comment