gorodenkoff/iStock via Getty Images
A little while ago, I wrote a cautious article on Nano-X Imaging Ltd. (NASDAQ:NNOX) summarizing the controversies surrounding the company. The company recently announced its Q2/2022 earnings results. Have there been any developments that would change my view?
Despite a recovery in the stock price to $16 a share, implying an $850 million market cap, there have been few fundamental developments for the company. The company is still waiting to submit its 510(k) submission to the FDA, and no actual shipments are expected in 2022. It has also started writing off an acquisition that was only completed months ago.
I think investors should stay away from the NNOX if they can; and if they own shares, they may want to consider using this stock rally to recoup their capital.
Second Quarter Was A Non-Event
The second quarter’s financial results were a non-event. NNOX reported revenues of $2 million and GAAP EPS of -0.38, both were less than analyst expectations. Revenue was generated from the sales of radiology services and AI solutions, at negative gross margins. A large part of the GAAP net loss of $19.6 million was due to a $14 million goodwill impairment charge related to the Nanox.AI unit. Recall, NNOX acquired Zebra Medical Vision (now Nanox.AI) in November 2021; to start writing down the acquisition within 3 quarters is fairly shocking.
Cash and marketable securities fell to $127 million, indicating a cash burn of $12 million in the quarter.
Waiting To Wait Some More
On the earnings call, CEO Erez Meltzer said in his prepared remarks:
I’d now like to discuss our progress on the regulatory front. Beyond the beneficial revenue streams, I just mentioned we made further progress in our efforts to gain FDA clearance for the Nanox.ARC multisource system. Recently, we received feedback from the FDA on our Q submission supplement that we previously submitted to the agency. The feedback we have received has been valuable and it’s helping us to optimize our 510(k) submission. Subsequently, we are now preparing our 510(k) submission to the FDA for our multisource Nanox.ARC, which we expect will have the full power levels and indications that we believe will result in a successful deployment of the Nanox.ARC. In parallel, we are preparing for global deployment of our multisource Nanox.ARC.
Recall, one of my key critiques on the NNOX story is the unending delay in its 510(k) submission to the FDA for the multisource Nanox. ARC scanner. From the CEO’s comments, it appears the company is now ‘preparing’ the submission. However, when asked about the actual FDA timeline, Mr. Meltzer’s response sounded non-committal:
With respect to the question that you asked about the FDA. First of all, I was very happy with the meeting that was held last week, which was actually the last — not the last, but the last meeting before the final preparation for this submission. I would say that the feedback that we received from the FDA was very helpful and meaningful for us. So as we say we are in the final preparation for the formal submission and it’s going to be aligned with our path moving forward.
Right now, I would say that the idea to go to the pre-sub into the Q submission and to take the advantage for — I would say, about eight almost eight months dialogue, continuous dialogue with the FDA and proven itself to be the right decision. And I think that all the feedback that we got and the cooperation that we are — that we had with FDA and we continue to have with the FDA will make it much easier for us to do it, so —
Further on the call, when another analyst tried to get clarification on the FDA timeline, CFO Ran Daniel also sounded vague and non-committal:
Suraj Kalia
Okay, fair enough. So, Ran, when — are you at — or can you give us a timeframe as to when the multisource 510(k) will actually be filed? And when you’re expecting clearance?
Ran Daniel
As you already know, I’m actually — I can say or indicate or willing to commit only things which are 100% sure that there will be on the time that I’m actually committing. So right now, I think that I gave enough background and information about the timeline. We are in the final preparation to the submission and it will be done shortly. And I think that the — this is — I would say, positively think the feedback that we got from the FDA gave us the advantage to add a few things in the submission that will be done in the next few weeks that will be added to the system. So I hope that this submission will be — will then shortly. And once it’s submitted, I’m sure that you’ll know about it.
Suraj Kalia
So you will file more than likely in Q4?
Ran Daniel
You said so? Basically, I’m always trying to do things earlier on all level and all fronts.
I simply cannot understand the difficulty in gathering the necessary supporting documents and file a submission to the FDA after 8 months of “dialogue.” Why can’t management even commit to file the submission before the end of the year?
NNOX Received More Orders From Africa
On the earnings call, management also revealed that NNOX had received an order from a Ghanaian distributor for 350 units for the multi-source Nanox.ARC scanner, bringing total contracted units to 68,150 worldwide. However, when analysts asked about whether the units will be shipped before year-end, management replied:
Right now, I would say that the — we explained in the past that basically not all the countries that we’re working in require the FDA regulation, like we mentioned, Nigeria, one of them and Ghana will be another one. We have another two that are not really related to the submission of the — to the formal approval of the FDA. Ghana has a special process that we are going through, they gave us indications, but I wouldn’t actually — until I see it in my eyes, I wouldn’t actually commit to — we hope to have the approval to send the systems during the fourth quarter. And I would say the starting gun next year, it will be probably more system that will be sent. Overall, the agreement is for 350 units.
From the response, it appears that although NNOX received the Ghanaian “orders,” there will be no actual shipment before year-end. In fact, with the product almost 2 years delayed, are the 68,000+ orders that were initially announced in 2020 still valid?
Insiders Have Sold More Shares
One aspect of the NNOX story that puzzled me was why haven’t insiders sold their shares, if the prospects for the company were truly poor? A search on most insider-trade monitoring websites has come up empty.
It appears I was looking at the wrong place. From sharp-eyed commentator Richard X Roe, “Executive officers and directors of foreign private issuers, such as Nanox, do not file Form 4 – they file Form 144 when they sell.”
Sure enough, a Google search of Nano-X Form 144 shows that Ran Poliakane, the founder and former CEO, had sold tens of millions of dollars’ worth of shares in early 2021 and several million dollars in June 2022.
Technicals Have Improved But Not Fundamentals
In my prior article, despite my scepticism on the company and product, I warned against shorting the stock:
At the same time, I would not short the stock at current levels, as a lot of the froth has been taken out of the stock, with it falling from $95 to $8.
Since my article, the shares have rallied on the back of improved market risk sentiment, almost doubling from the lows of ~$8 to over $16 recently (Figure 1).
A market cap of $850 million is even more expensive than when I last covered the stock, with little fundamental reason for the advance. Long-time shareholders may want to use this opportunity to sell their shares and recoup some capital at better prices.
Figure 1 – NNOX stock price has rallied (Author created with price chart from StockCharts.com)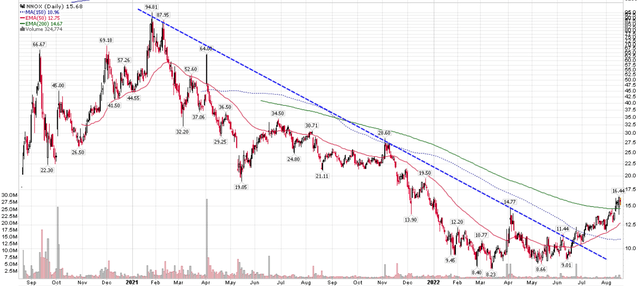
Conclusion Remains The Same
Despite a recovery in the stock price to $16 a share, implying an $850 million market cap, there have been few fundamental developments for investors to cheer about. The company still has not submitted the critical 510(k) submission to the FDA for them to begin the review of the multi-source Nanox.ARC scanner. In fact, management would not even commit to a submission-timeline before year-end 2022. At the same time, NNOX has announced additional orders from jurisdictions like Ghana that do not require FDA approval, but again, would not commit to any shipments prior to year-end. Finally, the company has begun writing down the AI acquisition that was completed only months ago.
In summary, my conclusion on NNOX remains the same. None of the red flags I have highlighted in my articles have been addressed, and we now know the founder had been selling tens of millions of dollars of shares. I think investors should stay away from the stock if they can; and if they own shares, they may want to consider using this stock rally to recoup some capital.


Be the first to comment